COVID-19 Taskforce approves vaccination for 3-11 year olds and expands vaccine choice for those aged 12-17
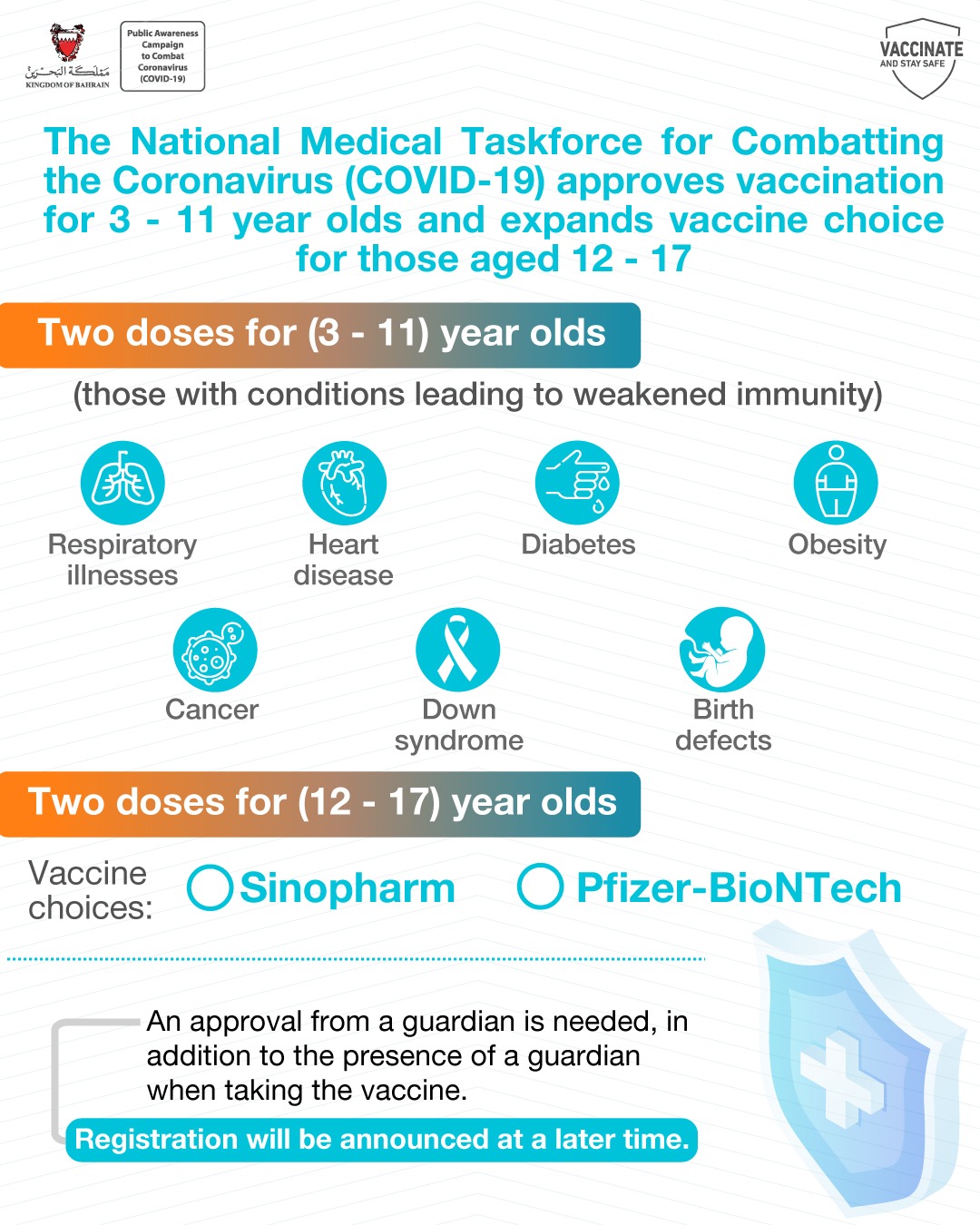
The National Medical Taskforce for Combatting the Coronavirus (COVID-19), today announced the Vaccination Committee’s approval of the vaccination of children aged 3-11 suffering from comorbidities and low immunity with the Sinopharm vaccine.
The decision, taken to safeguard public health, is based on a study that reviewed COVID-19 symptoms in patients suffering from diseases that cause weak immunity, including; respiratory illnesses, heart disease, diabetes, cancer, down syndrome, birth defects and obesity.
The Taskforce further noted the Vaccination Committee’s approval of the Sinopharm vaccine for those aged 12 to 17 years, in addition to the Pfizer-BioNTech vaccine.
The Taskforce stressed the importance of ensuring eligible children receive a vaccine to safeguard public health, adding that the incubation period for the virus in this age group without symptoms extends up to 4 days, which may increase the spread of the virus.
The Taskforce highlighted that vaccination registration for those 3-17 years of age will be available on the Ministry of Health’s website, healthalert.gov.bh, and via the “BeAware” application by selecting “Registration”.


